For a better understanding of how temperature and pressure influence air density, let's focus on a case of dry air. It contains mostly molecules of nitrogen and oxygen that are moving around at incredible speeds. Use our particles velocity calculator to see how fast they can move! For example, the average speed of a nitrogen molecule with a mass of 14 u (u - unified atomic mass unit) at room temperature is about 670 m/s - two times faster than the speed of sound!
Moreover, at higher temperatures, gas molecules further accelerate. As a result, they push harder against their surroundings, expanding the volume of the gas . And the higher the volume with the same amount of particles, the lower the density. Therefore, air's density decreases as the air is heated. Note that every molecule listed is heavier that or equal to 18 u.
Now, let's add some water vapor molecules to the gas with the total atomic weight of 18 u (H₂O - two atoms of hydrogen 1 u and one oxygen 16 u). According to Avogadro's law, the total number of molecules remains the same in the container under the same conditions . It means that water vapor molecules have to replace nitrogen, oxygen or argon. Because molecules of H₂O are lighter than the other gases, the total mass of the gas decreases, decreasing the density of the air too. The density of air is usually denoted by the Greek letter ρ, and it measures the mass of air per unit volume (e.g. g / m3).
Dry air mostly consists of nitrogen (~78 %) and oxygen (~21 %). The remaining 1 % contains many different gases, among others, argon, carbon dioxide, neon or helium. However, the air will cease to be dry air when water vapor appears.
The earth's atmosphere exerts a pressure, as does any other gas. Although we do not normally notice atmospheric pressure, we are sensitive to pressure changes—for example, when your ears "pop" during take-off and landing while flying, or when you dive underwater. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects .
Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure. In fact, normal air pressure is strong enough to crush a metal container when not balanced by equal pressure from inside the container. An online air density calculator such as the one by Engineering Toolbox let you calculate theoretical values for air density at given temperatures and pressures. The website also provides an air density table of values at different temperatures and pressures. These graphs show how density and specific weight decrease at higher values of temperature and pressure.
The density of air depends on many factors and can vary in different places. It mainly changes with temperature, relative humidity, pressure and hence with altitude . The air pressure can be related to the weight of the air over a given location. It is easy to imagine that the higher you stand, the less air is above you and the pressure is lower (check out our definition of pressure!). Therefore, air pressure decreases with increasing altitude.
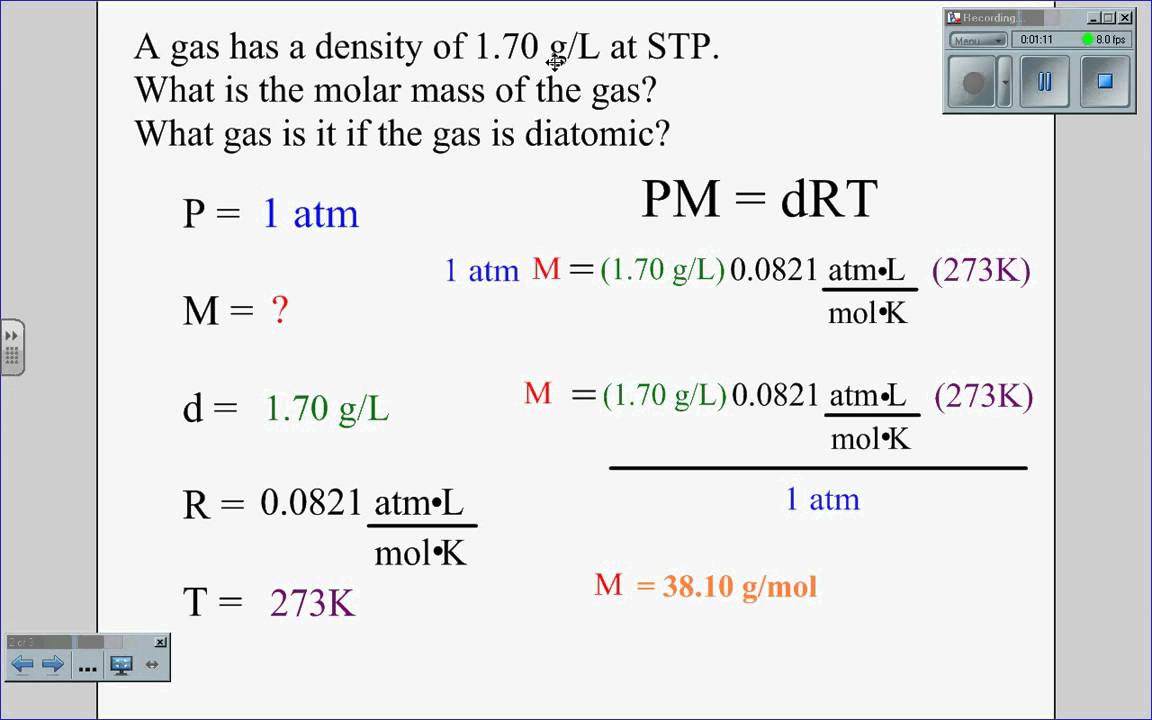
In the following text, you will find out what is the air density at sea level and the standard air density. A common use of Equation 10.23 is to determine the molar mass of an unknown gas by measuring its density at a known temperature and pressure. This method is particularly useful in identifying a gas that has been produced in a reaction, and it is not difficult to carry out. A flask or glass bulb of known volume is carefully dried, evacuated, sealed, and weighed empty. It is then filled with a sample of a gas at a known temperature and pressure and reweighed.
The difference in mass between the two readings is the mass of the gas. The volume of the flask is usually determined by weighing the flask when empty and when filled with a liquid of known density such as water. The use of density measurements to calculate molar masses is illustrated in Example 10.
Use this air density calculator to instantly find how tightly packed an object's molecules are, allowing you to estimate the ρ parameter basing on the local temperature and pressure conditions. This value is vital for many further calculations, such as determining the aerodynamic drag forces or the performance of wind turbines. Continue reading to get a better understanding of the relationship between the local weather and ρ, and learn what air density levels you can expect in various regions. The calculator below can be used to calculate the air density and specific weight at given temperatures and atmospheric pressure. For any ideal gas, at a given temperature and pressure, the number of molecules is constant for a particular volume (see Avogadro's Law). So when water molecules are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing.
Hence the mass per unit volume of the gas decreases. Is defined as a hypothetical gaseous substance whose behavior is independent of attractive and repulsive forces and can be completely described by the ideal gas law. In reality, there is no such thing as an ideal gas, but an ideal gas is a useful conceptual model that allows us to understand how gases respond to changing conditions. As we shall see, under many conditions, most real gases exhibit behavior that closely approximates that of an ideal gas. The ideal gas law can therefore be used to predict the behavior of real gases under most conditions.
As you will learn in Section 10.8 "The Behavior of Real Gases", the ideal gas law does not work well at very low temperatures or very high pressures, where deviations from ideal behavior are most commonly observed. Standard temperature and pressure are a useful set of benchmark conditions to compare other properties of gases. The ideal gas law can be used to determine densities of gases. If the air isn't completely dry, you can also add some water molecules (H2O) which are two atomic units for the two hydrogen atoms and 16 atomic units for the singular oxygen atom. If you calculate how much mass of air you have, you can assume that these chemical constituents are distributed throughout it uniformly and then calculate the percent of these chemical components in dry air. To get a better idea of the density of air specifically, you need to account for how air is made of different gases when formulating its density.
At a constant temperature, pressure and volume, dry air is typically made of 78% nitrogen (N2), 21% oxygen (O2) and one percent argon (Ar). The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables . Some applications are illustrated in the following examples. The approach used throughout is always to start with the same equation—the ideal gas law—and then determine which quantities are given and which need to be calculated. Let's begin with simple cases in which we are given three of the four parameters needed for a complete physical description of a gaseous sample. Dry air (0% humidity) is approximately 78% N2, 21% O2, and 1% Ar by moles.
Calculate the molar mass and density of dry air at 0 °C and 1.0 atm. Assume ideal behavior and use two significant figures in your answers. At constant pressure and volume, the density of a gas _____ as the molar mass increases. A gas made of larger particles will have a _____ density than a gas composed of smaller particles under the same conditions of temperature and pressure.
Atmospheric pressure is caused by the weight of the column of air molecules in the atmosphere above an object, such as the tanker car. At sea level, this pressure is roughly the same as that exerted by a full-grown African elephant standing on a doormat, or a typical bowling ball resting on your thumbnail. These may seem like huge amounts, and they are, but life on earth has evolved under such atmospheric pressure. If you actually perch a bowling ball on your thumbnail, the pressure experienced is twice the usual pressure, and the sensation is unpleasant. As you know, density is defined as the mass per unit volume of a substance. Since gases all occupy the same volume on a per mole basis, the density of a particular gas is dependent on its molar mass.
A gas with a small molar mass will have a lower density than a gas with a large molar mass. Gas density can be calculated from molar mass and molar volume. 15.Which one of the following statements is not consistent with the kinetic-molecular theory of gases?
The actual volume of the gas molecules themselves is very small compared to the volume occupied by the gas at ordinary temperatures and pressures. The average kinetic energies of different gases are different at the same temperature. There is no net gain or loss of the total kinetic energy in collisions between gas molecules. The theory explains most of the observed behavior of gases at ordinary temperatures and pressures. Calculate the number of molecules in a cubic meter of gas at standard temperature and pressure , which is defined to be 0ºC and atmospheric pressure.
At room temperatures, collisions between atoms and molecules can be ignored. In this case, the gas is called an ideal gas, in which case the relationship between the pressure, volume, and temperature is given by the equation of state called the ideal gas law. In which ρ is density in units of m/V mass/volume (kg/m3). Keep in mind this version of the ideal gas law uses the R gas constant in units of mass, not moles. Air - Specific Heat vs. Temperature at Constant Pressure - Online calculator with figures and tables showing specific heat of dry air vs. temperature and pressure.
The reaction of a copper penny with nitric acid results in the formation of a red-brown gaseous compound containing nitrogen and oxygen. A sample of the gas at a pressure of 727 mmHg and a temperature of 18°C weighs 0.289 g in a flask with a volume of 157.0 mL. Calculate the molar mass of the gas and suggest a reasonable chemical formula for the compound.
The density of gases have been listed below in alphabetical order in the units of both metric and imperial. The chemical formula as well as molar mass has also been listed. The properties of N2 will deviate more from ideality at -100oC than at 100oC. Van der Waal's equation corrects for the non-ideality of real gases.
Molecules of CH4 at high pressures and low temperatures have no attractive forces between each other. Molecules of an ideal gas are assumed to have no significant volume. Ρ is the object's density m is the object's total mass V is the object's total volume Under specified conditions of temperature and pressure, the density of a fluid is defined as described above.
However, the density of a solid material can be defined in several ways. Porous or granular materials have a density of the solid material, as well as a bulk density, which can be variable. For example, if you gently fill a container with sand, and divide the mass of sand by the container volume you get a value termed loose bulk density. If you took this same container and tapped on it repeatedly, allowing the sand to settle and pack together, and then calculate the results, you get a value termed tapped or packed bulk density.
Tapped bulk density is always greater than or equal to loose bulk density. In both types of bulk density, some of the volume is taken up by the spaces between the grains of sand. The density of the sand grains, exclusive of the air between the grains, will be higher than the bulk density.
The ideal gas law can be considered to be another manifestation of the law of conservation of energy . Work done on a gas results in an increase in its energy, increasing pressure and/or temperature, or decreasing volume. This increased energy can also be viewed as increased internal kinetic energy, given the gas's atoms and molecules. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Air pressure is a physical property of a gas that tells us with how much strength it has when acting on the surroundings. Let's consider a cubic container with some air closed inside.
According to the kinetic theory of gases, the molecules of the gas are in constant motion with a velocity that depends on the thermal energy. Particles collide with each other and with the walls of a container, exerting a tiny force on them. However, since the number of the enclosed molecules reaches about ~10²³ (order of magnitude of Avogadro's constant), the total force becomes significant and measurable - this is pressure. Argon - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of argon, Ar, at varying temperature and pressure - Imperial and SI Units. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Atoms and molecules are close together in solids and liquids.
Thus gases have lower densities than liquids and solids. Density is mass per unit volume, and volume is related to the size of a body cubed. So if the distance between atoms and molecules increases by a factor of 10, then the volume occupied increases by a factor of 1000, and the density decreases by a factor of 1000. The equilibrium vapor pressure of water is the pressure exerted by a vapor that is in thermodynamic equilibrium with its liquid phase at a given temperature.
It is a measure of the tendency of molecules or atoms to escape from a surface of a liquid and become a gas. As the temperature increases, the equilibrium vapor pressure increases too. Because of these dependencies and the fact that the Earth's atmosphere contains various gases the air density definition needs to be further expanded.
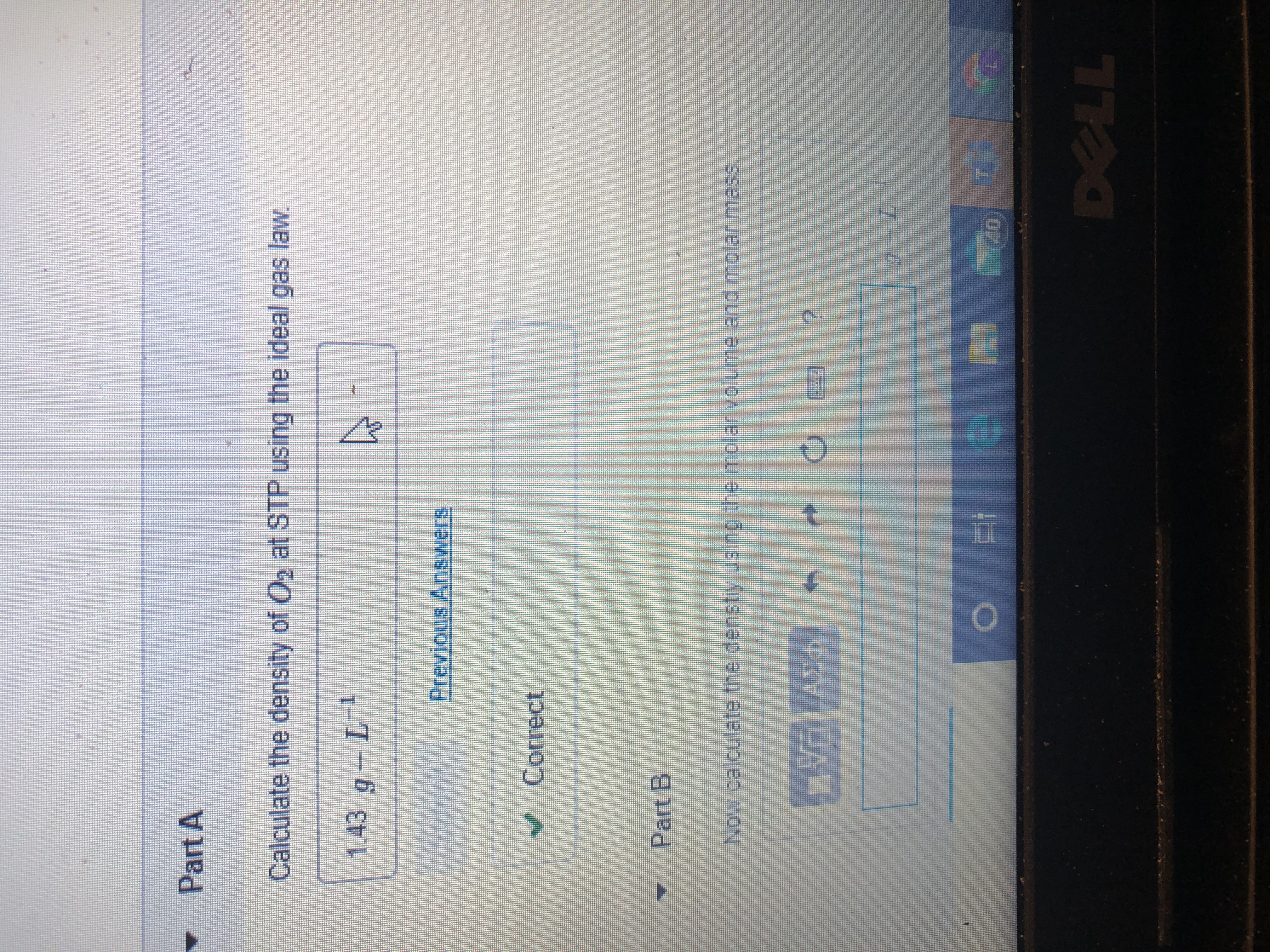
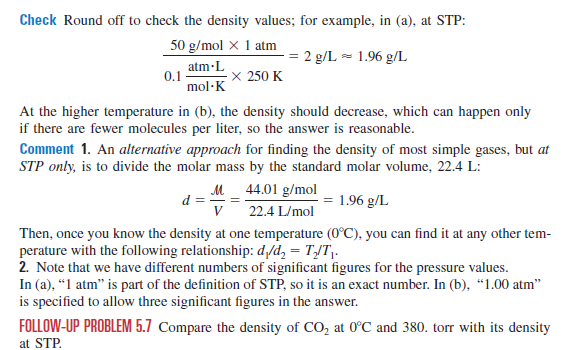
A proper modification has been made in our air density calculator with the density of air formula shown in the section called "How to calculate the air density?". To find the density of a gas, the volume and the mass must be known. At STP both of these quantities are known, as 1 mole of any gas occupies cm3. It is, therefore, a simple matter of dividing the molar mass by to obtain the density.
The values produced, however, are very small and gas densities are instead often quoted in grams per litre for this very reason. It is important to check your answer to be sure that it makes sense, just in case you have accidentally inverted a quantity or multiplied rather than divided. In this case, the temperature of the gas decreases. Because we know that gas volume decreases with decreasing temperature, the final volume must be less than the initial volume, so the answer makes sense. We could have calculated the new volume by plugging all the given numbers into the ideal gas law, but it is generally much easier and faster to focus on only the quantities that change.
Thus the ideal gas law does a good job of approximating the behavior of real gases at STP. We can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°C and 1 atmosphere pressure. As all gases that are behaving ideally have the same number density, they will all have the same molar volume. This is useful if you want to envision the distance between molecules in different samples. For instance if you have a sample of liquid water, it has a mass density of 1 g mL–1.
























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.